While the road to drug discovery and new treatment options for Lyme disease may seem slow, it’s imperative that the science is sound.
Lyme disease patients all know the thrill of hearing through the grapevine about a new remedy for chronic Lyme disease symptoms or a new diagnostic test. Often by word-of-mouth or through patient forums, a patient will hear that someone’s symptoms improved after taking an exciting new treatment. A savvy patient may then do a Google search on this new treatment option, perhaps discovering a few websites discussing the treatment, sometimes even finding a scientific research article that supports the other patient’s claim. Fantastic news, let’s talk to our doctor about it, or start our own regimen of treatment, if we can take something over-the-counter. Why not?
In actuality, this approach is not the best one, for many reasons. For one, the other patient may be taking a slew of other treatments, or be in a different stage of Lyme disease. Also, this may be a one-off or temporary result for this one patient, with most patients not responding to the same treatment. But what if you found results in a scientific journal that support the claim? Those results, if the studies were conducted in animals, may not be at a suitable dose or useful at all in humans, and perhaps even harmful.
While the road to new diagnostics and new treatment options for chronic Lyme disease symptoms and other illnesses may seem unreasonably sluggish, the science behind the diagnostics and treatments must be solid, and often that translates into a long process, but one that results in more successful treatments. To appreciate this at a deeper level, let’s step into what’s needed for good scientific research, and why clinicians and patients benefit from this process.
The gold standard for reporting scientific information is the peer-reviewed journal article, which is the cornerstone for high quality scientific research, and the best avenue for presenting information to the scientific community. These articles undergo a rigorous process in which other research specialists in the same field provide feedback, which the authors need to address by modifying their written content or experimental studies, or face rejection. For example, the researchers may need to return to the bench and add in some better controls, or even add in a whole new experiment before publishing. This powerful review process drives the most significant science because holes or weaknesses in the science are identified and can be weeded out before going to the next phase of research. Another perk is that these articles are a fantastic resource for organizations like NIH or GLA when they are deliberating over the most promising projects to fund. This iterative process of inquiry and criticism by knowledgeable specialists’ fuels quality and is necessary to achieve the most meaningful therapeutic, diagnostic or medical device.
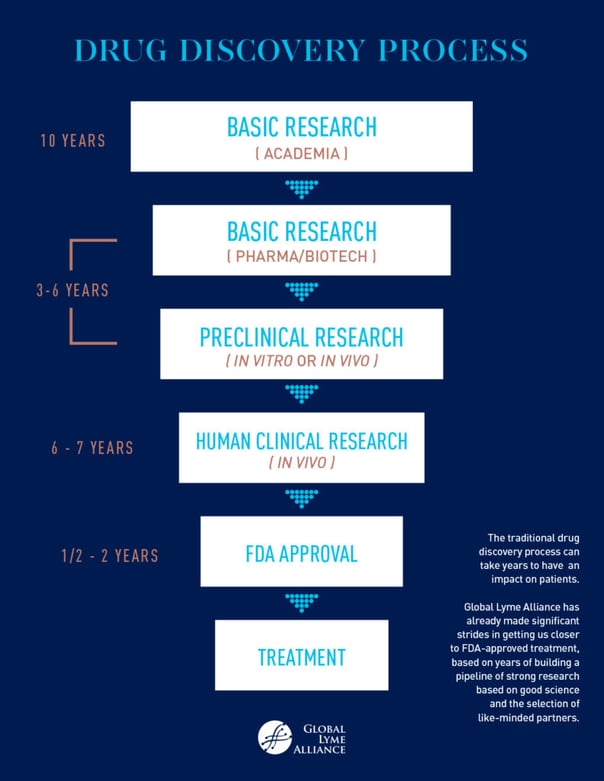
The drug discovery process is designed like a funnel, or pyramid, with a large amount of basic research at the outset, only some of which progresses into more applied research, culminating in a clinically useful therapeutic or diagnostic. Basic research has no immediate obvious application. Rather, it explains how things work, e.g., researchers have observed some limited evidence that Lyme disease bacteria, Borrelia burgdorferi, take on different forms besides that of spirochetes, which could theoretically protect the bacteria from antibiotic treatment. Often basic research starts by observing results in vitro (in a petri dish or in culture). Clinical research applies findings from basic research. It tests what interventions or medicines might be useful in improving treatments e.g., testing a therapeutic that works well against one of these alternative forms of B. burgdorferi. In clinical trials for a medical intervention, researchers start with preclinical studies on animals, then move into humans if they are successful. An in vivo preclinical study or series of studies on a novel therapeutic for Lyme disease might involve infecting mice with B. burgdorferi, observing and quantifying alternative bacterial forms, treating the mice, and looking at results.
The next step after preclinical studies, if they are successful, is to then move into human clinical trials. These human studies involve small trials to address preliminary questions such as safety and side effects (Phase I trials), and then gradually progress to larger trials involving more patients and an evaluation of efficacy (Phase III-IV). These trials might evaluate if patients reach a defined endpoint for efficacy after being treated with the novel therapeutic, such as a defined reduction in Lyme disease symptoms. Only if the intervention makes it through all of these stages does it get the opportunity to be reviewed, and possibly approved by the FDA (if U.S. based) for use in humans. Similarly to clinical research, translational research builds on basic research to create new therapies, medical procedures, or diagnostics, and moves the science from bench to bedside. This sometimes involves a multidisciplinary effort. For example, a fantastic way to implement the newly approved therapeutic would be to create a companion diagnostic that detects alternative forms of B. burgdorferi, thus screening for patients that would be suitable candidates for the novel treatment.
All of this research is generally very expensive to carry out. Large pharmaceutical and biotech companies will sometimes have their own pot of money to support some or all of these phases of development. In many cases, however, companies and academic organizations are highly dependent on grant money funded by the government or non-profits such as GLA. Early seed money from research grants investigates good ideas. If data is promising, researchers can apply for further funding for small scale grants for pilot studies, in which they can flesh out their ideas with more samples, more controls, and perhaps start evaluating the mechanism of action. Full-scale grants are provided for fully-realized studies, which involve proper controls, delineated milestones, building on previous work and data, and further mechanism of action studies. The NIH should be funding all levels of this research for Lyme and tick-borne diseases, but unfortunately, its support is not commensurate with case numbers.
Another embedded factor to implement high level research is the lengthy time and considerable cost in educating and training skilled researchers, involving training scholarships and fellowships. The traditional academic path requires a researcher to have a BS/BA, followed by a Ph.D. and a postdoc fellow position. The researcher then may go on to hold a junior faculty/early career investigator position and then may become an Assistant/Associate/Full Professor who leads an independent lab. Even entering pharmaceutical/biotech companies as a Ph.D.-level scientist sometimes requires postdoc fellow training, as competition is high for these positions. Supporting development of people on this pathway would keep them in the Lyme and tick-borne disease research community instead of losing them to areas of greater funding.
Yet another boon to fostering research, but adding to the expense, is the Lyme disease and tick-borne disease research community conferences, seminars etc. that help to foster networking, collaboration, and exchange of reagents and ideas.
This culture of inquiry, collaboration and constructive feedback is inherent and necessary for scientific discovery and application, and in the long run may produce top-notch therapeutics, diagnostics and treatments for patients, making it all worth the wait.
____
By Global Lyme Alliance and Dana Barberio, M.S.
____
Learn more about GLA’s research initiatives and accomplishments:
2018 Research Report
Published Research Findings
Current Grantees
First Observational Study for Lyme Disease Treatment

GLA
Admin at GLA




-2.jpg)

